Abstract
Background Graft-versus-host disease (GVHD) prophylaxis with post-transplant cyclophosphamide (PTCy), tacrolimus (TAC) and mycophenolate mofetil (MMF) for allogeneic haploidentical (haplo) donor hematopoietic cell transplantation (HCT) has proven safe and effective. Many centers replace TAC with sirolimus (SIR) as this drug preserves regulatory T cells and may have a preferable side effect profile with less risk of kidney injury and thrombotic microangiopathy. A recent phase II single arm study reported comparable rates of grade II-IV acute GVHD with the use of SIR after haplo HCT (Bejanyan, Blood Adv. 2021).
Methods We performed a retrospective cohort study comparing haplo HCT outcomes with SIR vs. TAC in combination with PTCy/MMF. All consecutive patients receiving haplo donor T cell replete peripheral blood stem cell graft HCT for hematologic malignancy at the Moffitt Cancer Center or the City of Hope Cancer Center between 2015-2020 were included. Associations with HCT related survival outcomes were assessed using Cox proportional hazard survival models. Fine and Gray regression models were used to assess associations of transplant related endpoints with competing risks. Kaplan Meier curves and cumulative incidence function curves were also plotted using the IBM SPSS analytic software version 28.
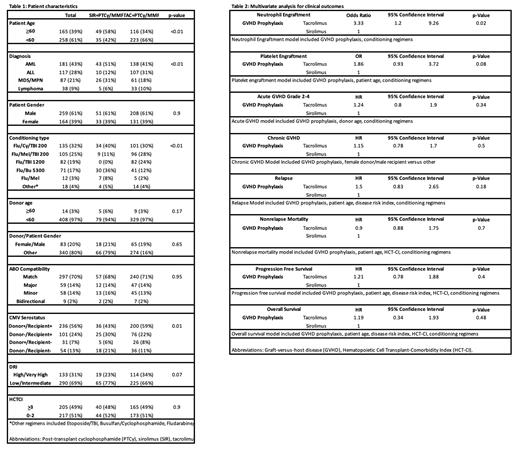
Results A total of 423 patients met the inclusion criteria of which 84 (20%) received SIR and 339 (80%) received TAC. The SIR group had a higher proportion of patients >60 years (58% vs. 34%, p=<0.01), and the groups were also unbalanced in terms of diagnosis type, conditioning regimen, and cytomegalovirus serostatus (Table 1). Median follow-up was 30 months (range: 2-88 months). Neutrophil engraftment at day 30 was lower with SIR than TAC (89% vs. 95%, p=0.04), while platelet engraftment at day 60 was not significantly different (77% in SIR vs. 84% in TAC; p=0.14). For SIR and TAC, we found no significant differences in the rates of grade II-IV acute GVHD (45% vs. 47%, p=0.6), grade III-IV acute GVHD (20% vs. 15%, p=1.0) or chronic GVHD (48% vs. 55%, p=0.80). The probability of non-relapse mortality (NRM) was similar between SIR vs. TAC groups both at day 100 (19% vs. 11%, p=0.16) and at 1 year (29% vs. 28%, p=0.98) after HCT. Similarly, we observed no significant differences between the SIR and TAC groups in 2-year probabilities of relapse (23% vs. 25%, p=1.0), disease free survival (DFS; 55% vs. 55%, p=1.0) or overall survival (OS; 64% vs. 63%, p=0.99). In multivariate analysis (MVA) (Table 2), TAC was associated with faster neutrophil engraftment (OR=3.33, CI 1.20-9.26, p=0.02), but GVHD prophylaxis type had no significant impact on platelet engraftment, acute or chronic GVHD, relapse, NRM, DFS, or OS after haplo HCT.
Conclusions Our study suggests that SIR is a comparable alternative to TAC in combination with PTCy/MMF for GVHD prophylaxis, resulting in overall similar clinical outcomes after peripheral blood haplo HCT. Rather than a uniform approach, the choice of TAC vs. SIR may be determined based on the side effect profile of these medications with consideration of patient medical comorbidities at HCT.
Disclosures
Elmariah:Bristol Myers Squibb: Research Funding. Ali:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Faramand:Kite/Gilead: Research Funding; Novartis: Research Funding. Fernandez:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Lazaryan:Teladoc: Current equity holder in publicly-traded company; AmWel: Current equity holder in publicly-traded company; Sanofi: Consultancy; AvroBio: Consultancy; Humanigen: Consultancy. Liu:Sanofi: Speakers Bureau. Nakamura:Sanofi: Consultancy; Magenta Therapeutics: Consultancy; BluebirdBio: Consultancy; Helocyte Inc: Research Funding; Omeros: Consultancy; Kadmon: Consultancy. Pidala:CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Johnson and Johnson: Research Funding; Pharmacyclcis: Research Funding; Abbvie: Research Funding; BMS: Research Funding. Marcucci:Abbvie: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings. Locke:CAREducation: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Society for Immunotherapy of Cancer: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; ASH: Other: Education or editorial activity; Leukemia and Lymphoma Society: Research Funding; Aptitude Health: Other: Education or editorial activity; ), National Cancer Institute: Research Funding; CERo Therapeutics: Research Funding; Takeda: Consultancy; Sana: Consultancy; BMS: Research Funding; Daiichi Sankyo: Consultancy; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Bejanyan:Medexus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CareDX Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Al Malki:Gilead: Consultancy, Research Funding; Miltenyi Biotec: Consultancy, Research Funding; NexImmune: Consultancy, Research Funding; Hasna Biopharma: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; CareDx: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal